Abstract
Introduction
Clinical outcomes of anti-CD19 chimeric antigen receptor T-cell (CAR-T CD19) therapy for the treatment of relapsed/refractory Diffuse Large B Cell lymphoma (r/r DLBCL) must be improved, as patients undergoing CAR-T CD19 therapy can expect a cure rate of only 41% and a median survival of just 6.3 months if unresponsive. Of patients presenting with naïve DLBCL, 35% will advance to third-line treatment with CAR-T CD19 therapy. Poor clinical responses to CAR-T CD19 therapy and a small window of survival for unresponsive patients highlights the critical need for improvement of CAR-T CD19 therapy in the setting of r/r DLBCL. Overexpression of anti-apoptotic Bcl-2 protein has been characterized in r/r DLBCL, and contributes to the disease's aggressive phenotype. Aberrant expression of the Bcl-2 protein proves targetable by an FDA-approved small molecule inhibitor which mimics the role of pro-apoptotic BH3 proteins, known as Venetoclax. Here, we investigate the effect of Venetoclax on CAR-T CD19 cell immunophenotype, effector function, and cytotoxicity upon co-culture with a lab-generated model of immortalized rituximab/chemo-resistant B cell lymphomas (Raji4RH) which recapitulate the resistant phenotype of r/r DLBCL seen in patients.
Methods
PBMCs were isolated and activated from apheresis samples from consenting adults (healthy donors) using the Miltenyi Biotec TransACT human CD3/CD28 Kit. Cells were transduced with lentiviral vectors encoding for 2nd generation CAR constructs 24 hours post-activation. Transduced cells were cultured in RPMI media supplemented with hIL-2 (1ng/mL) and hIL-7 (10ng/mL). At day 14, cells were cultured with Raji4RH and Venetoclax in either combined or solitary cultures. Co-culture cytotoxicity assays were performed by pre-labeling target cells with permeant dye (Thermo Fisher Scientific CellTrace Blue Proliferation Kit), then incubating with CAR-T CD19 cells (GFP labeled) at various effector:target ratios and Venetoclax concentrations for 24 hours. Sample wells were stained with viability dye and acquired on BD LSR II Cytometer. CAR-T CD19 cell immunophenotype was identified following 24-hour exposure of Venetoclax to CAR T cell populations at various concentrations. Following exposure, samples were stained with viability dye, CD3, CD4, CD8, CD45RA, CD45RO, CD62L, CCR7, CD25, and CD95. Frequencies of T REG, T Central Memory, T naive, and T Stem Cell Memory populations were calculated. CAR-T CD19 cell effector function was elucidated by activating (24 hours), then exposing CAR-T CD19 cells to concentrations of Venetoclax for 24 hours. Brefeldin A was added to culture for the remaining 4 hours of incubation. Cells were collected and stained intracellularly for IFNγ, Perforin, and Granzyme B; and extracellularly for CD3, CD4, and CD8. Sample acquisition for effector function assay was performed on BD LSRII Cytometer.
Results
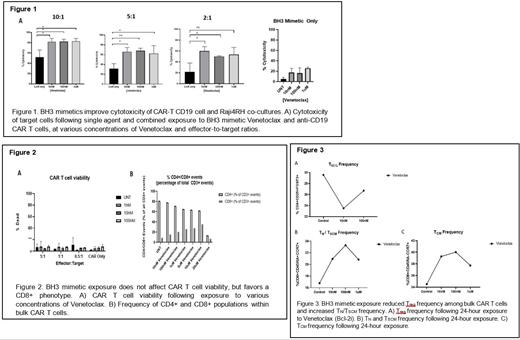
Concurrent in vitro CAR-T CD19 administration and Venetoclax exposure significantly increases Raji4RH cell death as opposed to single agent cohorts. CAR-T CD19 cell viability is unaffected by Venetoclax at concentrations which confer synergy, and a predominantly CD8+ population is favored. Finally, Venetoclax appears to significantly increase the frequency of T N/T SCM and T CM within exposed cultures, while affording a 21% decrease in T REG frequency. Additionally, the notable increase in T N/T SCM and T CM phenotypes following exposure to Venetoclax did not arise as a consequence of decreased cellular viability.
Conclusion
The combination of Venetoclax exposure and αCD19 CAR T cell administration demonstrates synergy when employed in the context of rituximab-resistant lymphoma cells, which may be explained by the effect of the BH3 mimetic's exposure on CAR-T CD19 cell immunophenotype. The ability to manipulate Bcl-2 protein expression within CAR-T CD19 cells affords insight into the role that the Bcl-2 family pathway plays within CAR-T CD19 cell biology. In addition to answering fundamental questions of CAR T cell biology, the combination of Venetoclax and CAR-T CD19 therapy may provide a solution to the observed clinical gap, in which CAR-T CD19 therapy alone cures less than half of all patients who advance to this third line treatment regimen.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal